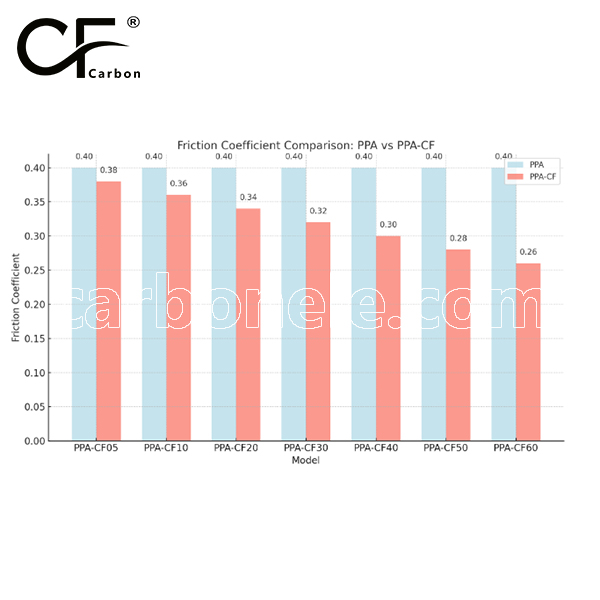
PEEK has a friction coefficient of 0.3 to 0.45, while PEEK-CF (carbon fiber reinforced) has a lower friction coefficient of 0.2 to 0.35, offering better wear resistance and reduced friction.

Durable PEEK-CF10 Polymer for Medical Implants
PEEK-CF10 carbon-reinforced medical polymer delivers strength & biocompatibility for spinal/orthopedic implants. MRI-compatible, lightweight, and chemically inert for enhanced patient outcomes
- Model number: PEEK-CF-BCA1
- Matrix Resin: Polyetheretherketone (PEEK)
- Reinforcing Filler: Carbon fiber
- Appearance: Granules
- Grade: Injection/extrusion grade
- Packaging: 25kgs/bag
Durable PEEK-CF10 Polymer for Medical Implants
Introduction to PEEK-CF10 in Advanced Medical Applications
The world of medical technology demands materials that can perform flawlessly under the most demanding conditions. In this pursuit, it has emerged as a breakthrough polymer offering unparalleled balance between strength, biocompatibility, and manufacturing versatility. Its ability to combine high mechanical stability with patient safety makes it an ideal candidate for the next generation of medical implants. With the ongoing evolution of surgical techniques and implantable device designs, it continues to set the standard for performance and reliability.
It is not just another engineering plastic. It represents a step forward in how modern polymers can be tailored to meet strict medical standards. The material integrates carbon fiber reinforcement into the PEEK matrix, delivering improved stiffness and dimensional stability without compromising the polymer’s inherent chemical resistance. These attributes are critical when developing implantable components that must endure bodily environments without degradation.
The Core Advantages of PEEK-CF10 for Medical Implants
In the medical sector, every choice of material carries life-altering consequences. The unique properties of PEEK-CF10 make it a standout option for applications that require both mechanical durability and biological compatibility.
Enhanced Structural Integrity
Medical implants constructed from PEEK-CF10 are known for their remarkable structural integrity. The reinforcement provided by the carbon fibers ensures that the polymer maintains its shape and functionality even under continuous physiological stress. Surgeons and device designers value this reliability because it translates to longer-lasting implants and fewer complications for patients.
Lightweight Comfort for Patients
One of the overlooked but vital aspects of implant materials is weight. A lighter implant made from PEEK-CF10 can significantly improve patient comfort while reducing strain on surrounding tissues. This is especially important in orthopedic implants, where excessive weight can influence mobility and healing outcomes.
Why PEEK-CF10 Stands Out in the Medical Field
The medical device industry is constantly comparing and evaluating material options. While metals such as titanium have dominated for decades, polymer-based solutions like PEEK-CF10 are now recognized for their distinct advantages.
Imaging Compatibility
Unlike certain metals, PEEK-CF10 does not interfere with medical imaging techniques such as MRI or CT scans. This makes postoperative monitoring more effective, allowing healthcare professionals to evaluate healing and implant performance without distortion.
Design Freedom for Complex Geometries
PEEK-CF10 can be processed through precision molding techniques, enabling the creation of complex shapes that would be difficult or expensive to achieve with metal. This design flexibility opens the door for custom implants that match patient anatomy more closely, improving surgical outcomes.
Application Example: PEEK-CF10 in Spinal Fusion Cages
One of the most impactful uses of PEEK-CF10 in the medical sector is its role in spinal fusion cages. These devices are inserted between vertebrae to support spinal stability and promote bone growth following injury or degenerative conditions. The choice of PEEK-CF10 for such implants is based on a combination of strength, radiolucency, and long-term biocompatibility.
Functional Benefits in Spinal Surgery
In spinal fusion, maintaining precise spacing and alignment between vertebrae is crucial. PEEK-CF10’s stiffness ensures that the cage maintains its dimensions under load. At the same time, its low weight reduces the risk of migration and minimizes stress on surrounding tissues.
Patient Outcomes
Patients with PEEK-CF10-based spinal cages benefit from improved post-surgical imaging clarity, which helps physicians track bone integration. The comfort provided by the lighter material can also support quicker rehabilitation timelines.
Manufacturing Precision with PEEK-CF10
Medical implants must be manufactured to exacting standards. PEEK-CF10’s consistent composition and thermal stability make it well-suited for advanced manufacturing processes.
Injection Molding Capabilities
The ability to injection mold PEEK-CF10 allows for repeatable, high-precision production runs. This is vital when producing implant components that must match exact specifications to integrate seamlessly into the human body.
Machinability for Custom Designs
While molding is ideal for mass production, PEEK-CF10 also offers excellent machinability. This makes it possible to create custom implants tailored to individual patients, an approach gaining popularity with advancements in 3D scanning and patient-specific surgical planning.
Sterilization Compatibility
Medical implants require rigorous sterilization before being introduced into the body. PEEK-CF10 retains its physical properties after exposure to various sterilization methods, including steam autoclaving and gamma irradiation. This durability across sterilization processes ensures that the material can be integrated into existing medical workflows without compromise.
Long-Term Stability in the Human Body
An implant’s success depends on its ability to function as intended for many years. PEEK-CF10’s resistance to hydrolysis, oxidation, and chemical degradation helps maintain its performance for the long term. This stability reduces the need for revision surgeries, improving patient quality of life and reducing healthcare costs.
Sustainability and Supply Chain Confidence
Although the focus for medical materials is often on patient outcomes, sustainability and supply chain reliability are also critical. PEEK-CF10 can be produced consistently to meet high-volume medical manufacturing demands while minimizing waste through efficient processing methods.
The Future of PEEK-CF10 in Medical Innovation
As surgical methods become less invasive and implants more specialized, the demand for materials like PEEK-CF10 will continue to rise. Its combination of strength, design flexibility, and patient safety aligns perfectly with the direction of modern medicine. Researchers and device manufacturers are exploring even more applications, from cranial plates to dental implants, leveraging the advantages of PEEK-CF10 to improve both function and aesthetics.
Conclusion
The durability, biocompatibility, and adaptability of PEEK-CF10 have established it as a leading choice for medical implants. Its ability to meet stringent performance requirements while offering advantages over traditional materials makes it an invaluable asset to surgeons, patients, and medical device manufacturers alike. As the field continues to evolve, it stands ready to support the next generation of medical breakthroughs.
If you want to get more information about PEEK-CF05, you can visit our YouTube.
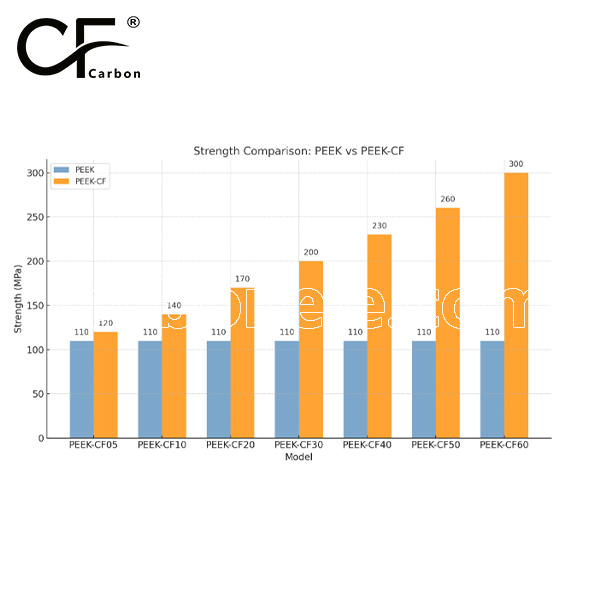
Strength between PEEK and PEEK-CF
PEEK offers good strength with a tensile strength of around 90-100 MPa, while PEEK-CF (carbon fiber reinforced) has significantly higher tensile strength (100-200 MPa) and flexural strength (150-250 MPa), providing greater stiffness and wear resistance. However, PEEK-CF may have slightly lower impact strength due to its brittleness from the carbon fiber reinforcement.

Frequently Asked Questions
Carbon (Xiamen) New Material Co., Ltd. aims to provide buyers with "one-stop" worry-free high-quality services. Here you can find all information about carbon fiber engineering plastics. If you still have questions, please send us an email for consultation!
-
How can I contact the manufacturer of a product that interests me?
When you find a product you are interested in, you can contact the manufacturer directly by sending an email and we will get back to you as soon as possible.
-
How do I find the products that interest me?
All you need to do is enter the keyword, product name in the search window and press the Enter key on your keyboard. Your search results page will then be displayed. You can also search within the product category pages on the home page. Each category is divided into subcategories, allowing you to refine your search and find products that interest you.
-
Where will I find a buying guide?
Please contact our after-sales service directly and we will provide you with a comprehensive operating guide.
-
What are CF Reinforced Thermoplastic Composites?
CF Reinforced Thermoplastic Composites are materials where carbon fibers are incorporated into a thermoplastic matrix. They combine the strength and stiffness of carbon fibers with the processability and recyclability of thermoplastics. For instance, they are used in automotive parts like bumper beams.
-
What are the benefits of CF Reinforced Thermoplastic Composites over traditional composites?
The key benefits include faster production cycles, easier recyclability, and better impact resistance. They also offer design flexibility. An example is in the manufacturing of consumer electronics casings where complex shapes can be achieved more easily.
-
How are CF Reinforced Thermoplastic Composites processed?
Common processing methods include injection molding, extrusion, and compression molding. Injection molding is widely used for mass production. For example, in the production of small components for the medical industry.
-
What industries use CF Reinforced Thermoplastic Composites?
They are utilized in aerospace, automotive, medical, and sports equipment industries. In aerospace, they can be found in interior components. In the medical field, they might be used in prosthetics.
-
How does the carbon fiber content affect the properties of the composites?
Higher carbon fiber content generally leads to increased strength and stiffness but may reduce ductility. A moderate content is often balanced for specific applications. For example, a higher content might be preferred in structural parts of a race car.
-
What are the challenges in using CF Reinforced Thermoplastic Composites?
Challenges include higher material costs, complex processing equipment requirements, and ensuring uniform fiber dispersion. Issues with adhesion between the fibers and the matrix can also arise. An example is in achieving consistent quality in large-scale production.